“`html
Effective Ways to Find the Limiting Reactant in Your Chemical Reactions
Understanding the concept of the limiting reactant is crucial when dealing with chemical reactions. The limiting reactant, often referred to as a restricting reactant, is the substance that is fully consumed when a reaction occurs. This article will explore effective methods for identifying the limiting reactant, engaging with relevant examples, and providing practical tips for accurate stoichiometric calculations.
Understanding the Theory of Limiting Reactants
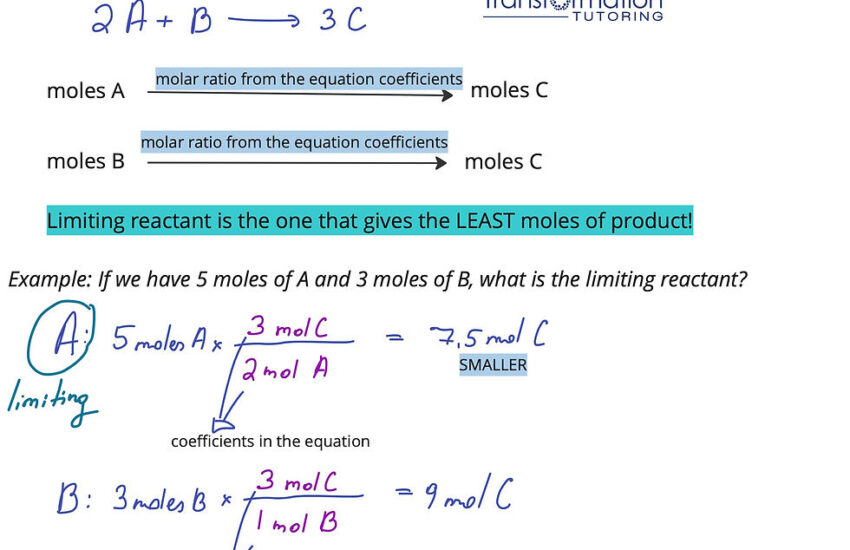
The theory of limiting reactants plays a fundamental role in reaction stoichiometry. At its core, this concept helps chemists determine which reactant will dictate when the reaction stops. When one reactant is used up, the reaction cannot proceed further, thus limiting the amount of product formed. This principle hinges on the mole ratio derived from balanced chemical equations.
Balanced Chemical Equations and Stoichiometric Ratios
A balanced chemical equation provides a foundation for all stoichiometric calculations. Each molecule’s coefficient represents the number of moles present. To find the limiting reactant, begin by writing and balancing the equation correctly. For example, consider the reaction:
2H2 + O2 → 2H2O
In this reaction, the mole ratio indicates that it takes 2 moles of hydrogen for every mole of oxygen. If you have 3 moles of H2 and only 1 mole of O2, hydrogen is in excess, and oxygen is the limiting reactant. Observing these relationships helps predict the total yield effectively.
Importance of Identifying the Limiting Reagent
Recognizing the limiting reagent is vital in various applications, from laboratory experiments to industrial processes. Accurately knowing which reactant is limiting allows chemists to optimize the reaction conditions for maximum reaction yield. For instance, excess reactants can lead to waste and increased costs in a chemical process. Proper identification contributes to better resource management and ensures that experiments yield consistent results.
Mathematical Approaches to Calculate Limiting Reactant
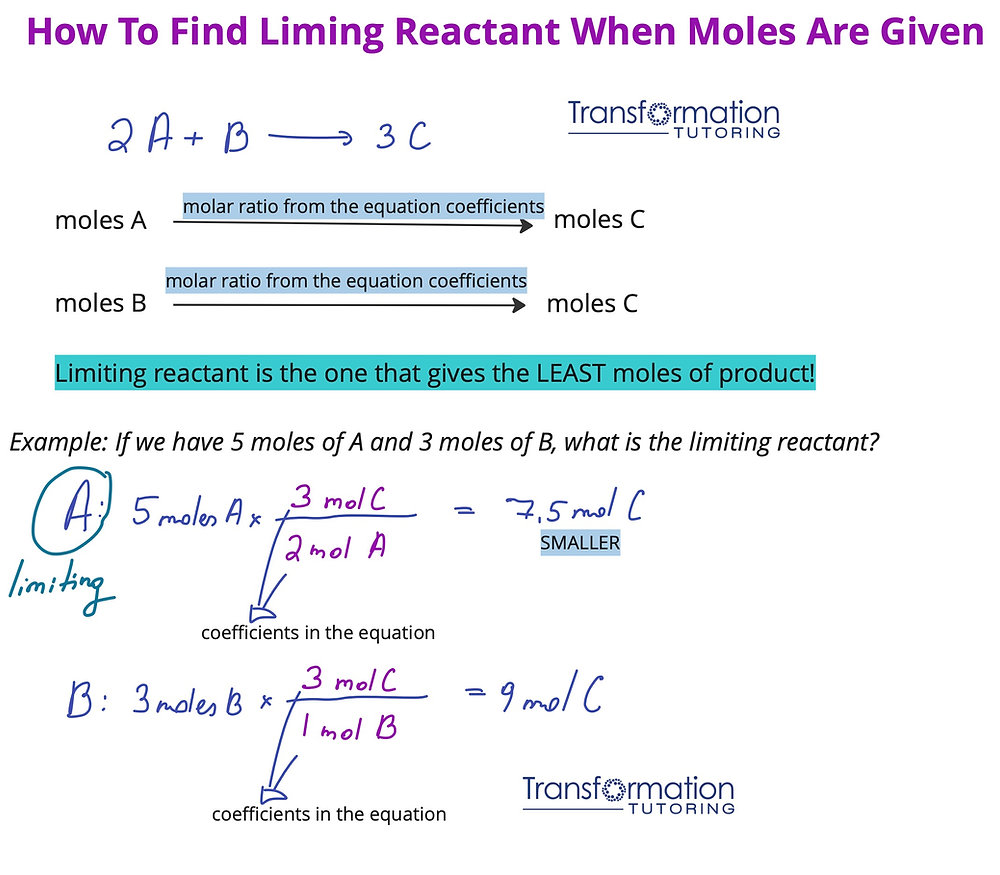
Utilizing a mathematical approach is one of the most effective ways to determine the limiting reactant. This method involves calculating the amount of product each reactant can produce and comparing the results.
Step-by-Step Calculation Method
To calculate the limiting reactant, follow these steps:
- Write the balanced equation for the reaction.
- Convert available amounts of each reactant to moles.
- Use the mole ratio from the balanced equation to determine how much product can be formed from each reactant.
- The reactant that produces the lesser amount of product is the limiting reactant.
As an example, when reacting 5 moles of nitrogen with 15 moles of hydrogen for the formation of ammonia (N2 + 3H2 → 2NH3), one can calculate the amount each reactant produces according to the balanced equation. Here, nitrogen would limit the reaction as it can produce 10 moles of ammonia while hydrogen contributes to 15 moles, claiming the additional hydrogen remains excess.
Common Stoichiometry Problems and Solutions
Various stoichiometry problems can arise. Understanding different scenarios aids in mastering the process of determining the limiting reactant. Some practical challenges include secondary reactions and impurities in reactants that may affect yield. In these instances, careful measurements and consideration of external factors can minimize errors.
Practical Applications of Limiting Reactants in Experiments
Insights from the study of limiting reactants extend far beyond theoretical knowledge. These principles are applied throughout various disciplines, enhancing laboratory techniques and facilitating chemical experimentation.
Real-Life Examples of Limiting Reactants
In numerous chemistry experiments, incorporating the concept of limiting reactants can optimize results. For example, in a reaction producing biodegradable plastics, understanding the limiting reagent will minimize waste and maximize efficiency. This stringent application can lead to cross-industry innovations in sustainable products, showing the power of chemistry in problem-solving.
Laboratory Techniques for Identifying Limiting Reactants
Effective laboratory techniques for identifying limiting reactants include titration and quantitative analysis. Using titration, chemists can determine the concentration of reactants, providing critical data for calculations. Moreover, implementing thorough experimental designs ensures results are accurate, considering the effects of temperature, pressure, and concentration on reaction rates.
Key Takeaways on Limiting Reactants
- The limiting reactant is the substance that is consumed entirely, dictating the end of a chemical reaction.
- Effective stoichiometric calculations require balanced equations and mole ratios.
- Mathematical and experimental methods provide the best strategies for identifying limiting reactants.
- Real-life applications of limiting reactants showcase the importance of optimization in chemical processes.
- Practical laboratory techniques enhance the understanding of reaction dynamics and efficiency.
FAQ
1. How do I know which reactant is in excess?
To identify the excess reactant, determine which compound neither fully reacts nor limits product formation. Calculate the amount of product each reactant can produce based on their initial amounts and balanced chemical equation. The one not limiting the amount of produced product is the excess reactant.
2. Can the limiting reactant change if conditions are altered?
Yes, the limiting reactant can change if conditions such as concentration, temperature or pressure are modified. For instance, increasing the amount of a previously limiting reactant may allow for a higher production yield, redirecting the role of the limiting reactant in that chemical reaction.
3. What are some practical limitations in reactions involving limiting reactants?
Practical limitations may include incomplete reactions, side reactions consuming reactants, or impurities in reactants affecting outcomes. A precise understanding of reactant concentrations and accurate balancing of equations helps mitigate these issues.
4. What is the importance of calculating the amount of excess reactant?
Calculating excess reactant amounts is crucial in maximizing resource efficiency and minimizing waste in chemical processes. Knowing how much excess remains informs future experiments and manufacturing processes, aiding in cost control and environmental sustainability.
5. How are limiting reactants relevant in real-life applications?
Limiting reactants are integral in industries from pharmaceuticals to plastics, guiding optimal yields and effective resource use. Understanding each chemical’s limiting role can enhance product quality control, ensuring higher compliance with industry standards.
For more details on the various aspects of limiting reactants and related chemical processes, check out the Limiting Reactant Cheat-Sheet and another guide on Stoichiometry Explained.

“`