Effective Ways to Calculate Percent Yield: A Smart Guide for Success in 2025
Understanding how to perform a **percent yield calculation** is crucial for scientists and students in chemistry. The ability to assess the efficiency of chemical reactions not only highlights practical applications but also influences the economic aspects of chemical manufacturing. In this guide, we will cover the **percent yield formula**, its importance, and how to effectively calculate it in various experiments.
Understanding the Percent Yield Formula
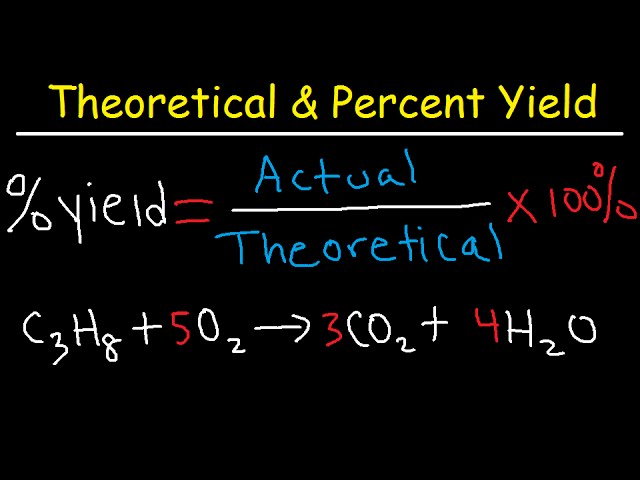
The **percent yield formula** plays a fundamental role in chemical experiments, providing insights into the efficiency of reactions. Percent yield is defined as the ratio of the actual yield to the theoretical yield, expressed as a percentage. Mathematically, it can be laid out as follows:
Percent Yield = (Actual Yield / Theoretical Yield) × 100%
Here, the **theoretical yield** is the maximum amount predicted based on stoichiometric calculations, while the **actual yield** represents the quantity obtained from an experiment. Understanding this formula is essential before diving into specific **percent yield examples** that demonstrate its application.
Why Percent Yield is Important in Chemistry
The application of **percent yield** in chemistry is significant as it dictates the success of a reaction in practical terms. Chemists aim to maximize their yields, meaning they seek to achieve a high **percent yield**, which indicates that a reaction proceeded efficiently with minimal waste. By calculating percent yield, chemists can determine how various factors, such as excess reactants or environmental conditions, affect their processes. This understanding leads to strategies for **improving percent yield**, thereby reducing costs and enhancing productivity in chemical production.
Percent Yield in Various Experiments
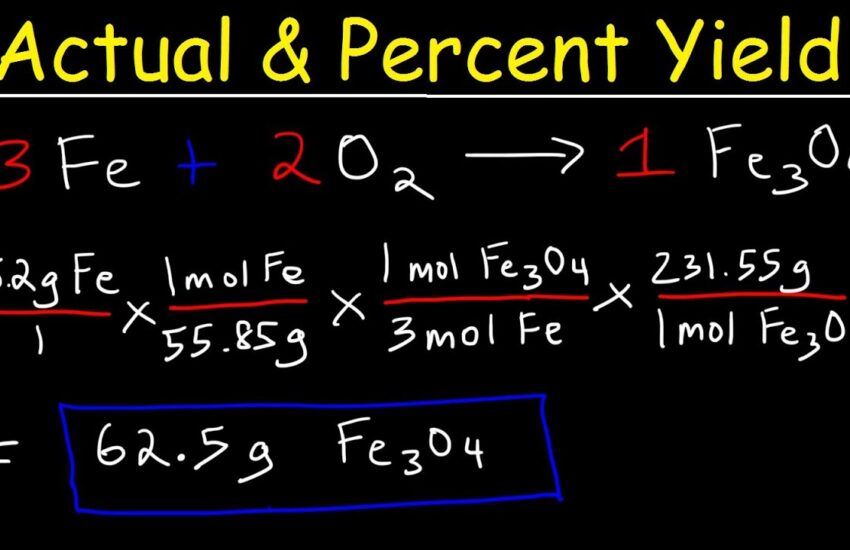
When conducting an experiment, calculating the percent yield helps evaluate the effectiveness of different methods. For instance, in a **percent yield lab** setup, students might explore various reaction pathways to determine their efficiencies. Consider a scenario involving the synthesis of an organic compound under different conditions. By comparing the percent yields under various circumstances, students can gain insights into how temperature, pressure, and reactant purity influence yield outcomes. This analysis is not only educational but also helps in practical scenarios such as drug development and industrial chemicals.
Calculating Percent Yield: Practical Steps
Knowing how to calculate percent yield methodically is essential for any chemist. Here’s a step-by-step approach for performing this **percent yield calculation** successfully.
Step-by-Step Percent Yield Calculation Method
To effectively calculate **percent yield**, follow these steps:
- Prepare Your Data: Gather information on the actual yield obtained from your reactions and the theoretical yield determined through calculations.
- Plug in Your Values: Substitute the actual and theoretical yield values into the formula: Percent Yield = (Actual Yield / Theoretical Yield) × 100%
- Perform the Calculation: Use a calculator to divide the actual yield by the theoretical yield, then multiply by 100 to get a percentage.
- Analyze the Result: Evaluate your percent yield result and consider any discrepancies or challenges encountered during the experiment.
Real-World Example: Calculating Percent Yield
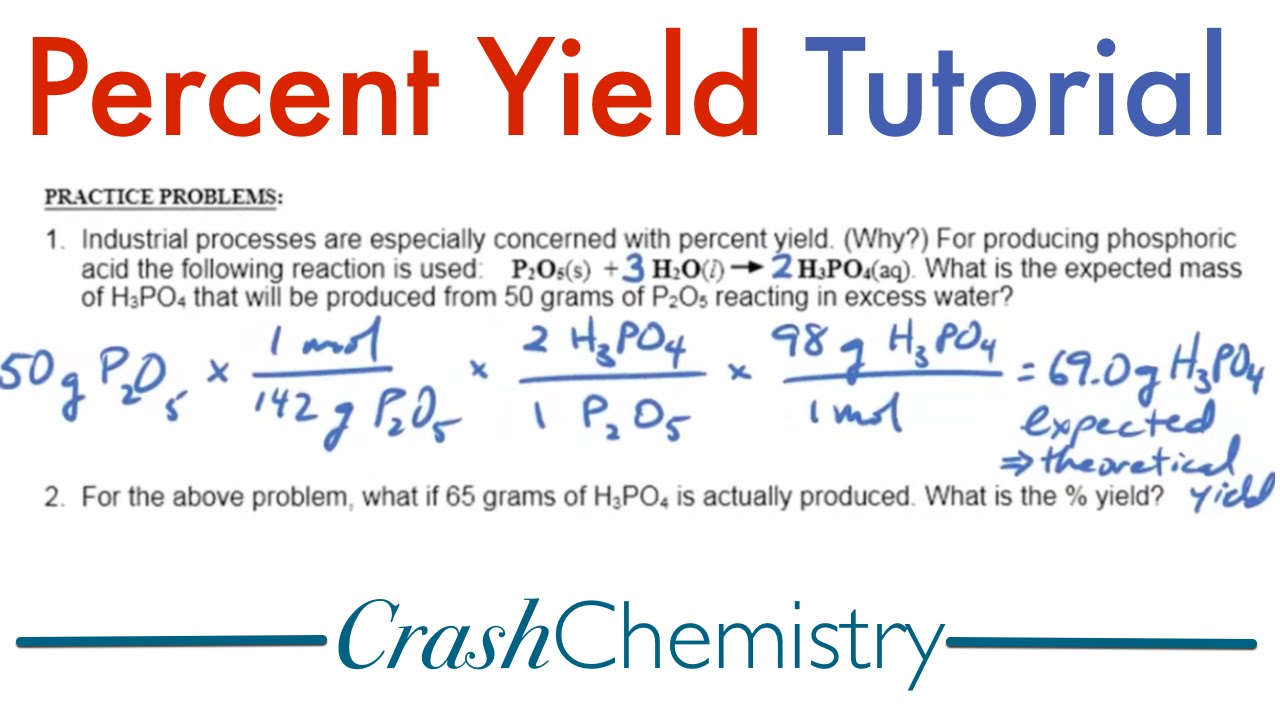
Let’s illustrate this with a practical example. Imagine conducting a reaction to synthesize water. Based on balanced equations, the theoretical yield for 100 grams of reactants might predict 88 grams of water. If your experimental results yielded 75 grams of water, the calculation would be:
Percent Yield = (Actual Yield / Theoretical Yield) × 100% = (75 g / 88 g) × 100% ≈ 85.23%
This yields an efficiency of about 85.23%, indicating a robust reaction, while also pointing to possible areas for improvement.
Common Factors Affecting Percent Yield
Several factors can influence **calculating percent yield** correctly. These variables play crucial roles in determining the outcome of a chemical reaction and can alter the **percent recovery** noted during experiments.
Impacts of Reaction Conditions
Factors such as temperature and concentration can significantly affect the percent yield achieved in experiments. For instance, increasing the temperature may enhance reaction rates but might also lead to unwanted side reactions, consequently affecting the **actual yield**. Maintaining optimal conditions can be critical to maximizing the output from reactions.
Purity and Reactant Quality
The quality of the **reactants** used in a reaction also plays a role in determining percent yield. Higher purity often leads to a more favorable yield. If impurities are introduced, they may consume reactants or produce side products which negatively affect the efficiency of a reaction.
Importance of Percent Yield in Chemical Reactions
Understanding percent yield is fundamental not only for academic purposes but for industrial applications as well. Insights derived from **yield calculations** can guide manufacturers in optimizing production processes, thereby reducing costs and waste. Furthermore, accurate yield analyses can facilitate the development of better methodologies for **yield in industrial processes**.
Assessing Chemical Yield Importance
The importance of understanding percent yield extends into broader aspects of chemical engineering and sustainability practices. By knowing how to properly conduct **percent yield determinations**, industries can enhance their evaluation processes, leading to vital improvements in environmental impact, cost efficiency, and overall effectiveness in chemical manufacturing.
Improvement Strategies for Percent Yield
Various techniques can enhance **percent yield** outcomes systematically. Implementing better quality control to ensure **high-yield reactions** is one strategy, along with employing advanced technology to monitor and optimize conditions thoroughly. Additionally, error reduction measures in laboratory settings can minimize discrepancies, ensuring results align more closely with theoretical predictions.
Key Takeaways
- Understanding and calculating **percent yield** is vital for evaluating the efficiency of chemical reactions.
- High percent yields indicate more efficient reactions, with broader implications for cost reduction and sustainability.
- Factors such as environmental conditions and reactant purity significantly affect yield outcomes.
- Practical, step-by-step methods can facilitate accurate percent yield calculations, improving experimental reliability.
- Enhanced techniques and quality assurance measures can bolster yield performance in various applications.
FAQ
1. What is percent yield in chemistry?
Percent yield in chemistry refers to the measurement of the efficiency of a given reaction, calculated as the ratio of actual yield to theoretical yield. This percentage indicates how much of the expected product was actually obtained under controlled conditions.
2. How do you distinguish between actual yield and theoretical yield?
The actual yield reflects the amount of product obtained from a chemical reaction, while the theoretical yield represents the maximum possible amount based on stoichiometric calculations. The difference between these two yields can help determine the effectiveness of a reaction.
3. Why is the determination of percent yield important in industrial processes?
In industrial processes, determining percent yield is critical for assessing production efficiency and cost-effectiveness. High yield metrics indicate optimizations that result in less waste and greater profitability, crucial for competitive industries.
4. What affect does temperature have on yield in chemistry?
Temperature can greatly influence yield in chemical reactions as it can increase reaction rates, but also may lead to unwanted side reactions. Understanding the optimal temperatures for various reactions can improve yield outcomes significantly.
5. Can percent yield be over 100%?
No, percent yield cannot exceed 100% in a theoretically ideal scenario. A yield above this value suggests that there might be measurement errors, side products formed, or additional factors that have inflated the result, such as evaporated solvents binding to products.
For more information on the methodologies for calculating yield, visit our additional resources: here and here.