How to Properly Calculate Moles for Accurate Chemical Analysis in 2025
Understanding how to calculate moles is essential for anyone engaging in chemistry, whether for academic purposes, industrial applications, or research. Moles serve as a fundamental unit that helps chemists relate the macroscopic amounts of substances to their molecular and atomic levels. In this article, we will explore various methods of mole calculation, examine key concepts such as converting moles to grams, and delve into the practical applications of moles in specific chemical situations. Let’s get started on this comprehensive journey to mastering how to calculate moles effectively.
Molecular Weight and Molar Mass Calculation
The foundation of understanding moles lies in grasping the concept of molecular weight and molar mass calculation. The molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is calculated by summing the atomic masses of all atoms in the molecular formula of the substance. For example, the molar mass of water (H₂O) can be calculated as follows: 2 hydrogen atoms (approximately 1 g/mol each) + 1 oxygen atom (approximately 16 g/mol) equals 18 g/mol. This molar mass serves as a crucial conversion factor in determining the number of moles present in a given mass or vice versa.
How to Find Molar Mass
Finding the molar mass is straightforward once the molecular formula is known. Firstly, look up the atomic weights of each element from the periodic table. Use the molecular formula to determine how many atoms of each element are present. For example, in ethene (C₂H₄), there are 2 carbon atoms and 4 hydrogen atoms. The molar mass calculation proceeds like this: (2 x 12.01 g/mol for carbon) + (4 x 1.008 g/mol for hydrogen) = 28.05 g/mol. Understanding this process is vital for conversions involving moles in chemistry.
Common Mole Calculations
Once you determine the molar mass, you can easily perform common mole calculations. For instance, converting grams to moles is commonly needed in laboratory settings. The formula is straightforward: number of moles = mass (in grams) / molar mass. So, if we have 36 grams of water, we can calculate the moles by dividing 36 g by the molar mass of water (18 g/mol), which results in 2 moles of water. This simple formula enables chemists to switch between mass and moles proficiently.
Moles in Solutions: Molarity and Molality
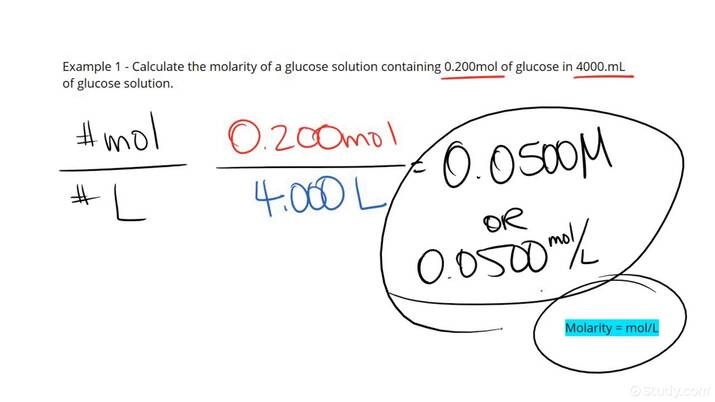
Moles are fundamental in understanding solutions, with molarity and molality being key concepts. Molarity (M) defines the concentration of a solution in moles of solute per liter of solution (mol/L), while molality (m) measures the concentration in moles of solute per kilogram of solvent (mol/kg). Both measurements are crucial when conducting reactions, preparing solutions, or performing titrations. For accurate stoichiometry and moles, it is essential to use the correct definition tailored to the needs of the procedure.
Understanding Molarity
Molarity is often utilized in laboratory settings as it provides a clear measure of concentration. To calculate molarity, one needs to dissolve a known quantity of the solute in a specified volume of solvent. For example, dissolving 5 moles of sodium chloride (NaCl) in enough water to make a 2-liter solution gives a molarity of 2.5 M (5 moles / 2 L = 2.5 M). Understanding moles in solutions via molarity is critical for accurately following chemical equations and protocols.
Molarity vs. Molality Usage
Choosing between molarity and molality depends on the experiment’s requirements. In temperature-sensitive reactions, molality is preferred as it’s less affected by temperature changes compared to molarity. For instance, suppose you have a reaction occurring at varying temperatures. Using molality ensures that you accurately account for changes in solvent volume while maintaining constant solute concentration. Knowledge of these differences aids in selecting appropriate methods for mole conversion in laboratory scenarios.
Stoichiometry and Moles in Chemical Reactions
Stoichiometry is the part of chemistry that deals with the relationships between reactants and products in chemical reactions. Applying stoichiometry and moles facilitates the understanding of how substances interact quantitatively. Using a balanced chemical equation, chemists can predict how much of each reactant is needed and how much product will be formed, all based on mole ratios derived from the coefficients in the balanced equation.
Finding Moles in Reactions
To find out how many moles of a substance participate in a reaction, one must start with a balanced chemical equation and use known quantities of the available substances. For instance, in the reaction that forms water, 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water (2H₂ + O₂ → 2H₂O). This stoichiometric relationship allows a chemist working backwards from the amount of water produced to determine the amount of hydrogen and oxygen initially required. This is fundamental in maintaining accuracy in calculating moles from volume or mass in experimental practices.
Calculating Moles from Volume Using the Ideal Gas Law
The Ideal Gas Law (PV=nRT), which relates pressure, volume, temperature, and moles, is instrumental in calculating moles in gases. Here, P is the pressure, V is the volume of gas, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. For example, if we have 10 liters of gas at 1 atm pressure and 300 K temperature, we can rearrange the law to find n: n = PV/RT. Substituting in the values gives us the ability to accurately quantify the number of moles in physical chemistry and gas laws.
Applications of Moles in Different Chemical Fields
Having a solid grasp of moles unveils various applications across different branches of chemistry, from biological systems to industrial processes. Chemists utilize moles in organic chemistry to quantify reactions, while analysts rely on precise mole calculations to inform tests and results in environmental science. Furthermore, moles feature prominently when exploring moles in thermodynamics, and various industrial applications demonstrate the relevancy of understanding moles in daily biochemistry or pharmaceutical engineering.
Moles in Academic Research and Industry
A comprehensive understanding of moles is leveraged both in academic research and industry applications. Molar fractions, the conversion of grams to moles calculation, and the significance of Avogadro’s number whilst preparing experimental designs or lab reports. As research progresses, calculations involving moles take center stage, enhancing analytical results and experimental accuracy throughout chemical analysis. For chemists, accurate mole conversions can be pivotal in determining the reliability of research findings and scientific conclusions, fostering advancements within diverse chemical fields.
Real-World Examples of Moles Usage
Real-world applications further demonstrate the vitality of moles—environmental chemistry utilizing moles to determine polluters, forensic investigations where moles guide evidence interpretation, and developing pharmaceuticals, necessitating rigorous conversions between moles and other measurements. The proficiency in making these calculations translates into enhanced safety standards, better product efficacy, and innovations across a multitude of scientific, engineering, and safety standards.
Key Takeaways
- Understanding how to calculate moles is essential for accurate chemical analysis.
- Molar mass plays a crucial role in converting mass to moles and vice versa.
- Molarity and molality are key for solution calculations and laboratory applications.
- Stoichiometry allows for predictions in chemical reactions based on mole relationships.
- Knowledge of moles has significant real-world applications spanning various industries and academic fields.
FAQ
1. What is Avogadro’s number, and why is it essential for mole calculations?
Avogadro’s number, approximately 6.022 x 1023, represents the number of particles (atoms, molecules, etc.) in one mole of a substance. It is essential as it allows chemists to convert between moles and the actual number of entities present, making it fundamental to understanding relationship between moles and molecules.
2. How do I convert grams to moles accurately?
To convert grams to moles accurately, you first need to determine the molar mass of the substance. The formula to use is: number of moles = mass (in grams) / molar mass (in g/mol). This approach can be quickly applied in experiments involving moles in solutions or common mole calculations.
3. What are some common mistakes in mole calculations?
Some common mistakes include using the wrong molar mass, not balancing chemical equations when calculating moles, or forgetting to account for unit conversions. Ensuring accuracy in datasets or calculations is crucial for the reliability of results, especially in academic and research settings involving moles in physical chemistry.
4. Can moles be used in environmental science?
Yes, moles find applications in environmental science, such as measuring concentrations of pollutants in water samples or air quality assessments. Moles allow for precise conversions involving moles which lead to more accurate data analysis and better insights into environmental conditions and regulations.
5. How do I utilize moles for solution preparation?
To prepare a solution, you first calculate the required number of moles of solute based on the desired molarity and the volume of the solution. Use the formula: number of moles = molarity x volume (in liters). After calculating moles, you can convert it to grams using molar mass for the necessary quantity of solute.